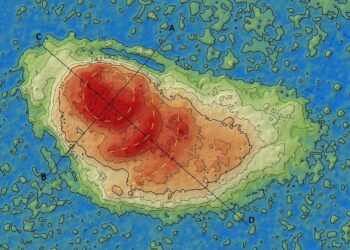
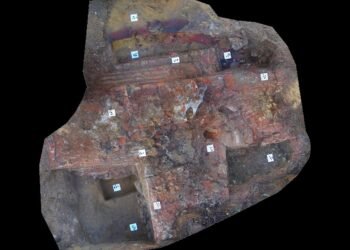
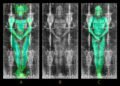
Accelerator Mass Spectrometry, or AMS, measures isotopes in extremely small quantities. Archaeologists often use this method to measure radiocarbon, known as carbon 14, in ancient organic remains. The technique builds on standard mass spectrometry but reaches far higher sensitivity. Researchers turn to AMS when samples contain only trace amounts of carbon-14.

Radiocarbon dating forms a core tool in archaeology. Living plants and animals absorb carbon from the atmosphere. After death, carbon 14 decays at a steady rate, with a half life of 5,730 years. Scientists compare the remaining carbon 14 in a sample to stable carbon isotopes such as carbon 12. From this ratio, they calculate the time since death.
Earlier radiocarbon methods relied on detecting radioactive decay events. Laboratories used liquid scintillation counting or proportional gas counting to measure emissions from carbon 14. These techniques required larger samples, often several grams of material. When dealing with a small bone fragment or a rare manuscript, removing that much material posed a problem. Error ranges also tended to be wider.
AMS takes a different approach. Instead of counting decay events over time, the instrument counts individual carbon 14 atoms. Technicians first convert the sample into graphite. The machine then ionizes the material and accelerates the ions to high energy. Magnetic and electric fields separate isotopes according to mass and charge. Detectors measure the ratio of carbon 14 to carbon 12 and carbon 13 with high precision.

Small sample size stands out as one of the main strengths of AMS. In many cases, laboratories need only a few milligrams of carbon. A single seed, a tiny charcoal fragment, or a sliver of parchment often provides enough material. This reduces damage to artifacts and allows dating of items once considered too small for analysis.
Precision improves as well. Because AMS counts atoms directly, age estimates often carry smaller statistical uncertainty. Laboratories also compare results with calibration curves built from tree ring records and other dated archives. These curves account for past changes in atmospheric carbon-14 levels, which have varied over thousands of years.
Careful preparation of samples plays a central role in reliable results. Technicians clean materials and remove contaminants such as soil carbon or conservation chemicals. This process limits outside carbon from altering the measured isotope ratio.
Researchers apply AMS beyond radiocarbon dating. By measuring isotopes of carbon and nitrogen in human and animal bones, scientists study diet and migration. Differences in isotope ratios reflect food sources such as marine fish or terrestrial plants. In this way, AMS supports research on population movement and long-term cultural patterns.
« Back to Encyclopedia Index